Addressing Key Challenges in Pharmaceutical Development
Discover how our expert consultation tackles industry hurdles with tailored, compliance-focused strategies.
GMP Compliance Expertise
We guide your operations to meet rigorous GMP standards, ensuring product safety and regulatory approval.
Product Registration Strategy
Our team crafts effective registration roadmaps to accelerate your pharmaceutical products’ market entry.
Market Research and Strategic Planning
Through detailed market analysis, we deliver actionable insights that optimize your business growth and marketing access.


Discover Our Expert Consultation Services
This section describes the key features briefly.
Pharmaceutical Industrial Consultancy
Design, development, and operation of pharmaceutical plants (Solid, Semi-solid, Injectables, BFS, MDI, Eye Drops).
Technical and financial feasibility studies.
Regulatory Affairs & Compliance
Product registration in UAE, Saudi Arabia, Iraq, and international markets.
CTD dossier preparation and EU/GCC regulatory representation.
Investment Advisory & Market Access
Market research and ROI analysis for pharmaceutical projects.
Structuring joint ventures (JV) and strategic partnerships across GCC and MENA.
Research & Development (R&D) Support
Market research and ROI analysis for pharmaceutical projects.
Structuring joint ventures (JV) and strategic partnerships across GCC and MENA.
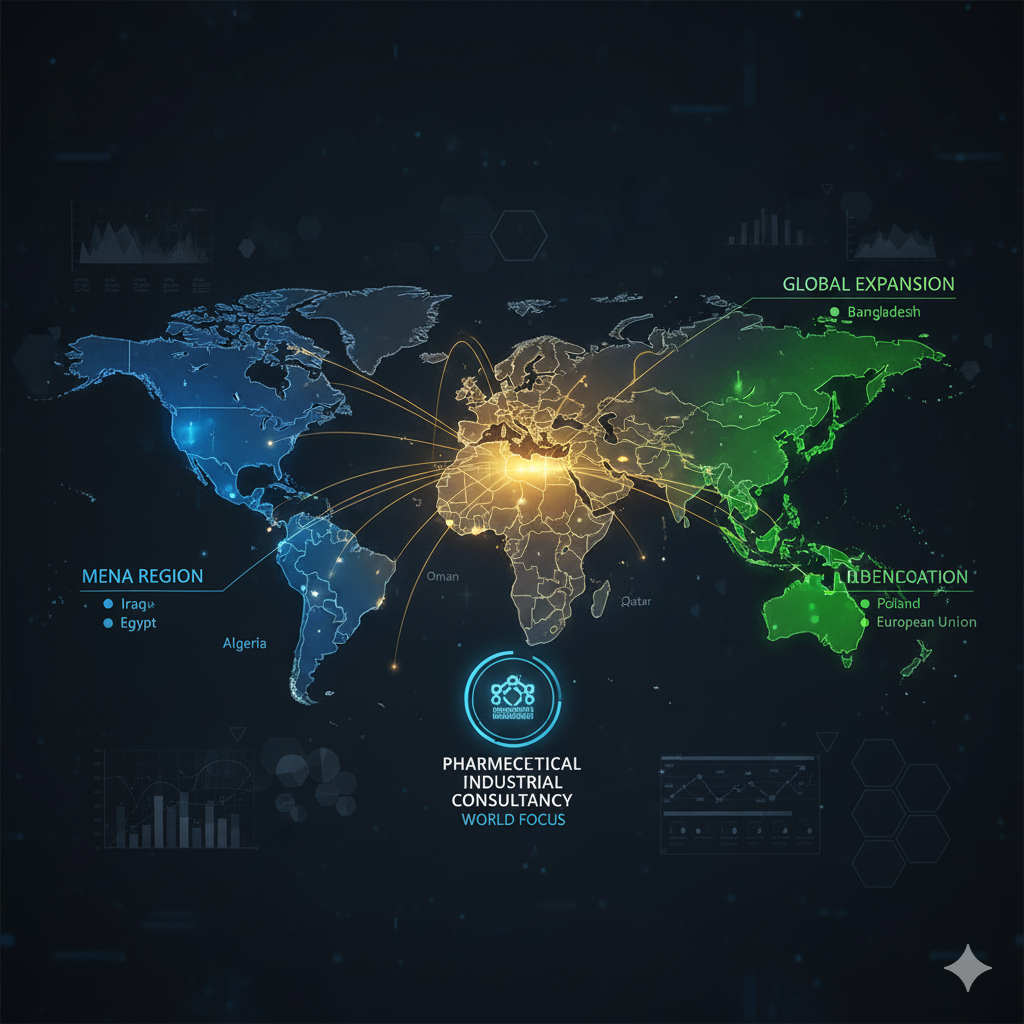
Markets We Serve
GCC Region: UAE, KSA, Oman, Qatar
• MENA Region: Iraq, Egypt, Algeria
• Global Expansion: Bangladesh, Poland, European Union

